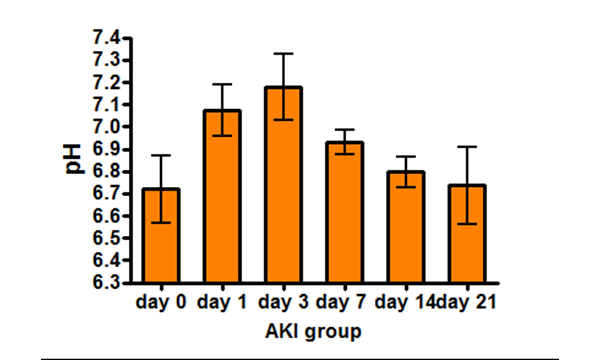
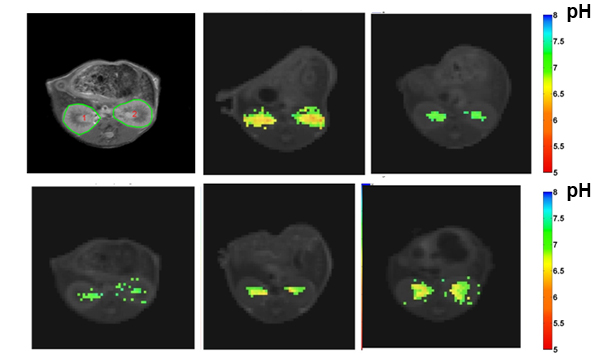
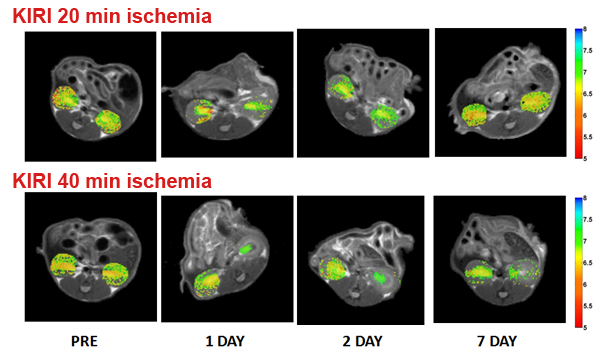
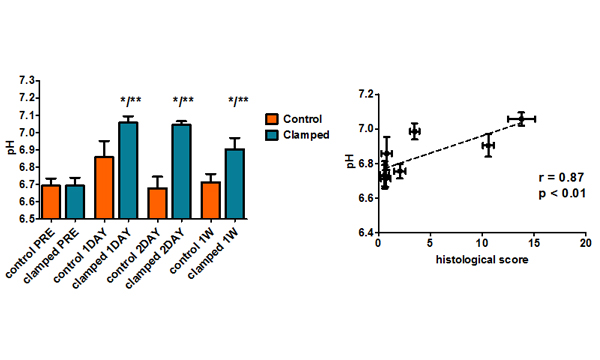
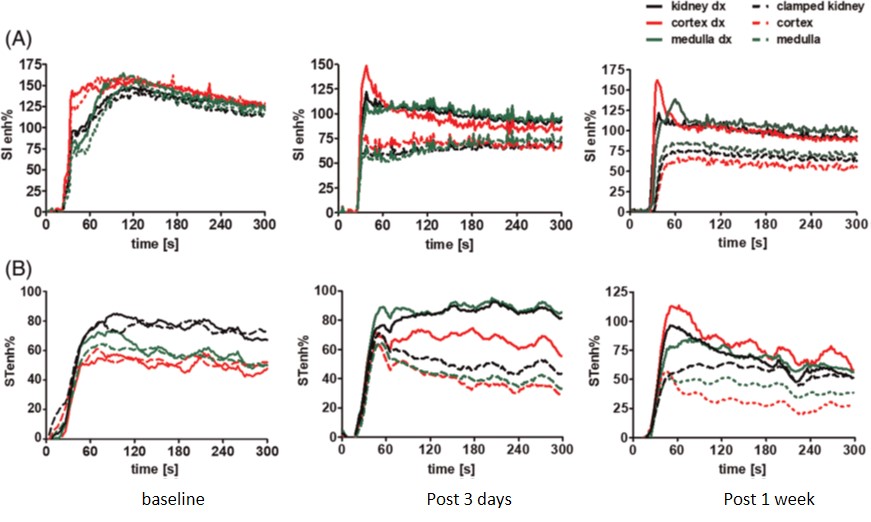
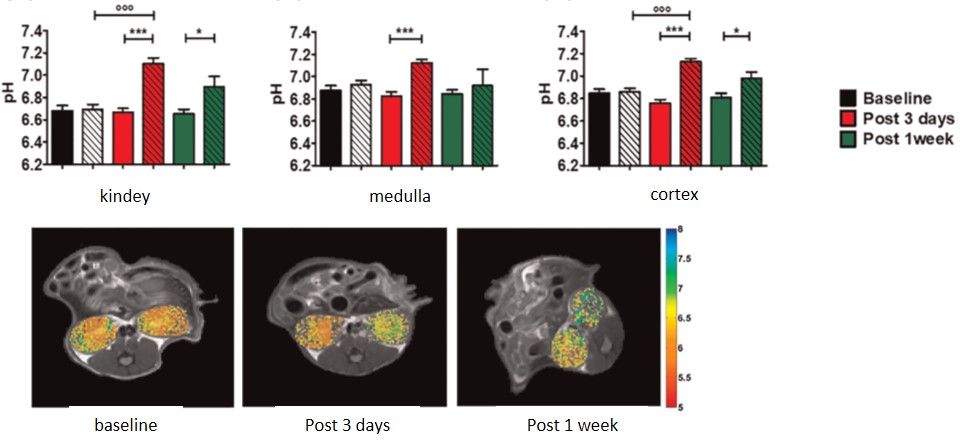
Imaging renal pH
The kidney is a highly complex organ consisting of welldefined
structures that function in a deeply coordinate
fashion to allow for fine regulation of pH homeostasis.
The role of the kidney in acid-base balance depends
on the capacity of the renal tubule to reclaim filtered
bicarbonate and to excrete net protons as titratable acids
and ammonium.
Although the principal role of the kidney is the maintenance of
acid–base balance, current imaging approaches are unable to
assess this important parameter and clinical biomarkers are not
robust enough in evaluating the severity of kidney damage.
Therefore, our lab is developing novel noninvasive imaging approaches
to assess the acid–base homeostasis in vivo and to monitor pH evolution following kidney injury.
Our lab is also actively involved in the COST Action "PARENCHIMA"
CA16103
(Magnetic Resonance Imaging Biomarkers for Chronic Kidney
Disease).
We aim to demonstrate the biological validity
of renal pH imaging as a novel biomarker of kidney diseases.