Apoferritin and other biological carrier for the delivery of imaging and therapeutic agents
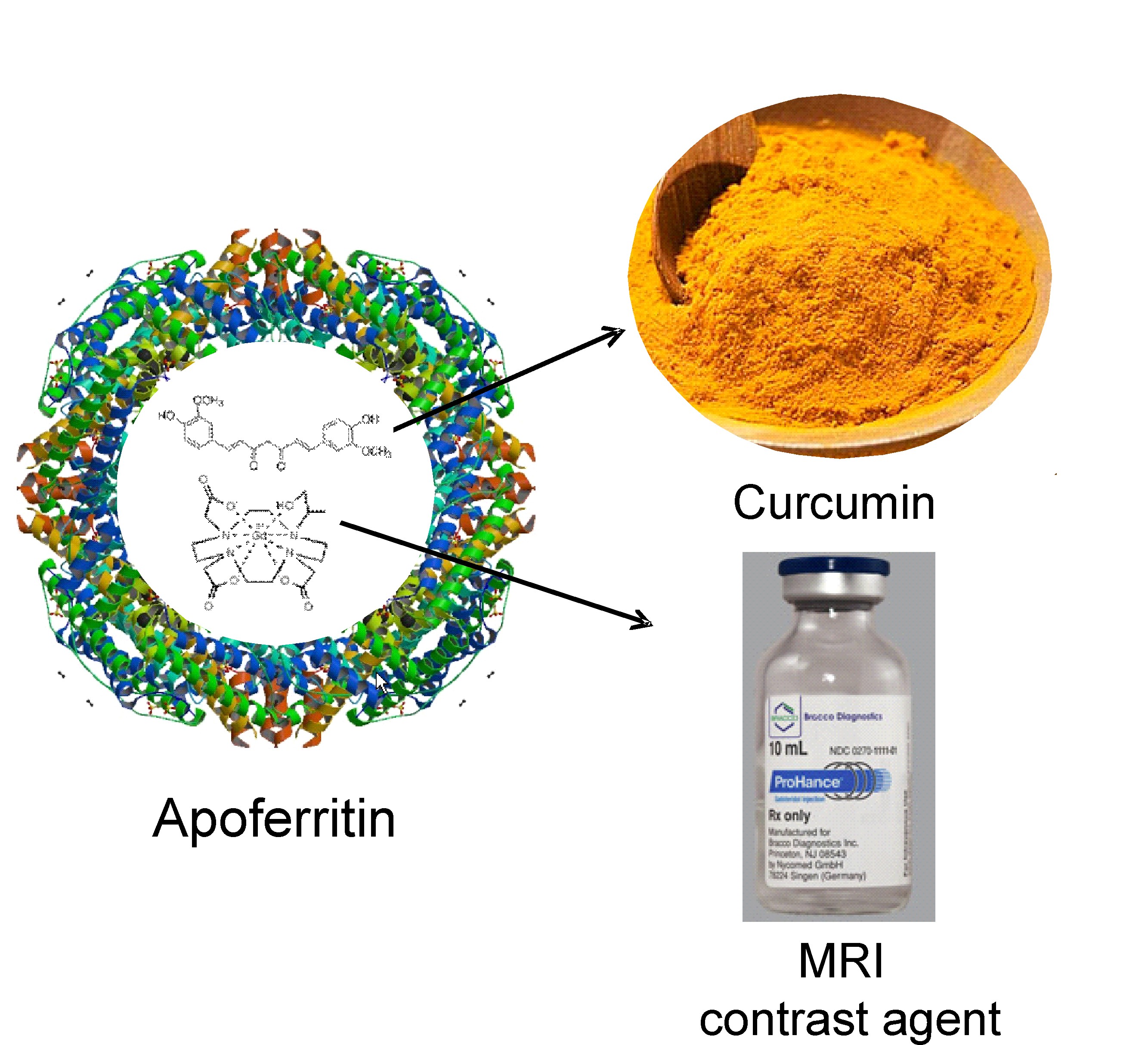
Apoferritin, a 24 subunits nanocage of H- and/or L-chain peptides forming a cage of 12 nm in external diameter with an interior cavity of 8 nm is an ideal drug- and imaging agent delivery platform due to its biocompatibility, biodegradability, coupled with its low toxicity.
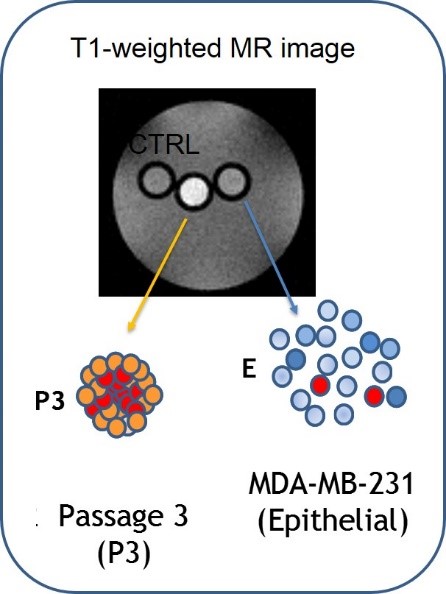
L-Ferritin targets breast cancer stem cells.
A growing body of evidence suggests that cancer stem cells (CSC) have the unique biological properties necessary for tumor maintenance and spreading, and function as a reservoir for the relapse and metastatic evolution of the disease by virtue of their resistance to radio- and chemo-therapies. Thus, the efficacy of a therapeutic approach relies on its ability to effectively target and deplete CSC. In this study, we show that CSC-enriched tumorspheres from breast cancer cell lines display an increased L-Ferritin uptake capability compared to their monolayer counterparts as a consequence of the upregulation of the L-Ferritin receptor SCARA5.
L-Ferritin internalization was exploited for the simultaneous delivery of Curcumin, a natural therapeutic molecule endowed with antineoplastic action, and the MRI contrast agent Gd-HPDO3A, both entrapped in the L-Ferritin cavity. This theranostic system was able to impair viability and self-renewal of tumorspheres in vitro and to induce the regression of established tumors in mice.
Conti L. et al, Oncotarget. 2016 Oct 11; 7(41): 66713–66727Curcumin and Gd loaded apoferritin: prevent Hepatic damage in Acute Hepatitis.
Apoferritin has been exploited to deliver simultaneously therapeutic and imaging agents (loaded into its internal cavity) to hepatocytes as this protein is efficiently taken up from blood by hepatocyte scavenger receptor class A type 5 via the ferritin transporting route. To this purpose the protein has been loaded with the magnetic resonance imaging (MRI) contrast agent GdHPDO3A and curcumin, a polyphenolic substance endowed with multiple pharmacological actions, namely: antioxidant, anti-inflammatory, antineoplastic. Curcumin and GdHPDO3A loaded apoferritin has been used with the aim to attenuate the thioacetamide-induced hepatitis together with the evaluation by MRI of drug delivery efficiency. Mice pretreated by intraperitoneal administration showed significantly attenuated hepatic injury as assessed by measuring alanine aminotransferase (ALT) activity in plasma and by histology assessment.
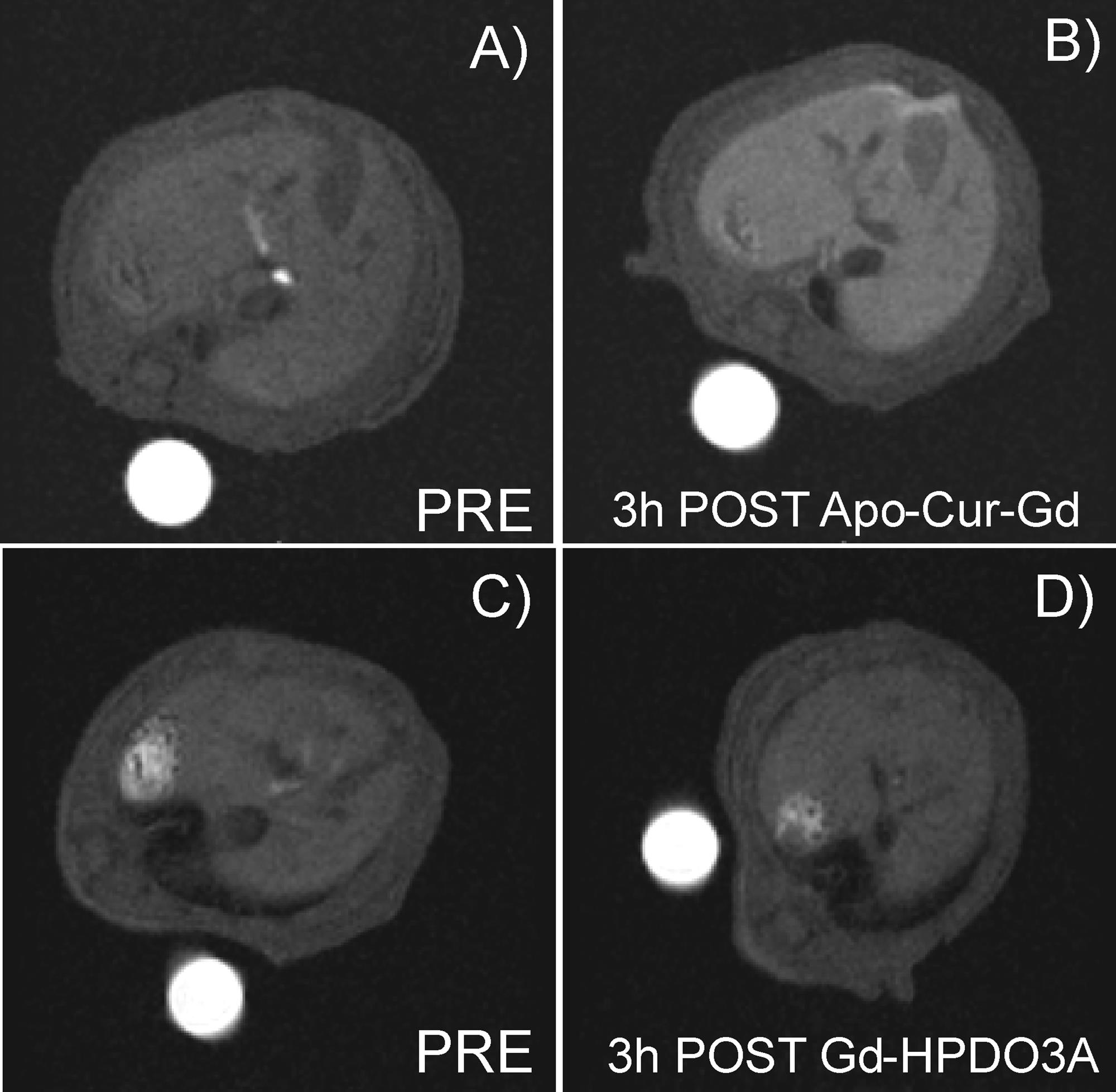
The encapsulation of curcumin inside the apoferritin cavity significantly increases its stability and bioavailability while maintaining its therapeutic anti-inflammatory properties.
Cutrin JC et al, Mol Pharm. 2013 May 6;10(5):2079-85.Visualization of Tumor Angiogenesis by Targeting Neural Cell Adhesion Molecules with the Highly Sensitive Gd-Loaded Apoferritin Probe.
Tumor vessel imaging could be useful in identifying angiogenic blood vessels and in the follow-up of antiangiogenic treatment response. We recently reported the expression of the neural cell adhesion molecule (NCAM) in the immature and tumor endothelial cell (TEC) lining vessels of human carcinomas.
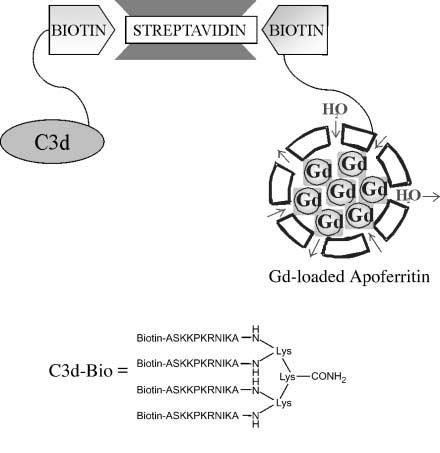
Exploiting an in vivo model of human tumor angiogenesis obtained by implantation of TEC in SCID mice, we aimed to image angiogenesis by detecting the expression of NCAM with MRI. The imaging procedure consisted of (a) targeting NCAMs with a biotinylated derivative of C3d peptide and (b) delivery of a streptavidin/gadolinium (Gd)-loaded apoferritin 1:1 adduct at the biotinylated target sites. The remarkable relaxation enhancement ability of the Gd-loaded apoferritin system allowed the visualization of TEC both in vitro and in vivo when organized in microvessels connected to the mouse vasculature. Gd-loaded apoferritin displayed good in vivo stability and tolerability. The procedure reported herein may be easily extended to the magnetic resonance visualization of other epitopes suitably targeted by proper biotinylated vectors.
Geninatti Crich S. et al, Cancer Res September 15 2006 (66) (18) 9196-9201