Design of targeting imaging probes
Active targeting is one of the most used approaches in pharmaceutical field in order to improve the concentration of a molecule (therapeutics or in vivo diagnostics) at the pathological site. Therefore, a targeting agent is a molecular system containing the "active principle" (a drug or an imaging probe) conjugated to a vector that has to exhibit a high affinity and specificity towards the biological target (cellular receptors, intracellular markers, enzymes, metabolite, extracellular components, ...). Moreover, the success of the approach is also based on the accessibility of the biological target by the targeting system. The design of a targeting imaging probe is affected by the sensitivity of the imaging modality. For instance, to overcome the relatively low sensitivity in the contrast detection of MRI, the targeting vector is conjugated to macromolecular or nanosized systems loaded with a high number of imaging units in order to significantly increase the contrast delivered at the biological target. On the contrary, in case of Optical or Nuclear imaging, sensibility is much better and a single fluorophore/radioisotope can be sufficient to generate enough signal from the disease marker. Considering the accessibility issue, MRI targeting agents are very suitable for endothelial markers that do not require the extravasation of the "large" probe, whereas optical probes can easily reach extracellular and intracellular targets.
MRI visualization of inflammation by paramagnetic micelles decorated with an anti VCAM-1 peptide.
This activity deals with the synthesis of mixed micelles composed by DSPE-PEG2000 and the amphiphilic Gd(III) complex GdDOTAMA(C18)2. The micelles were decorated with a cyclic peptide known to have a high affinity towards the endothelial receptor VCAM-1, which is overexpressed in presence of inflammation. In order to be loaded into the micelles the peptide was conjugated to a phospholipid.
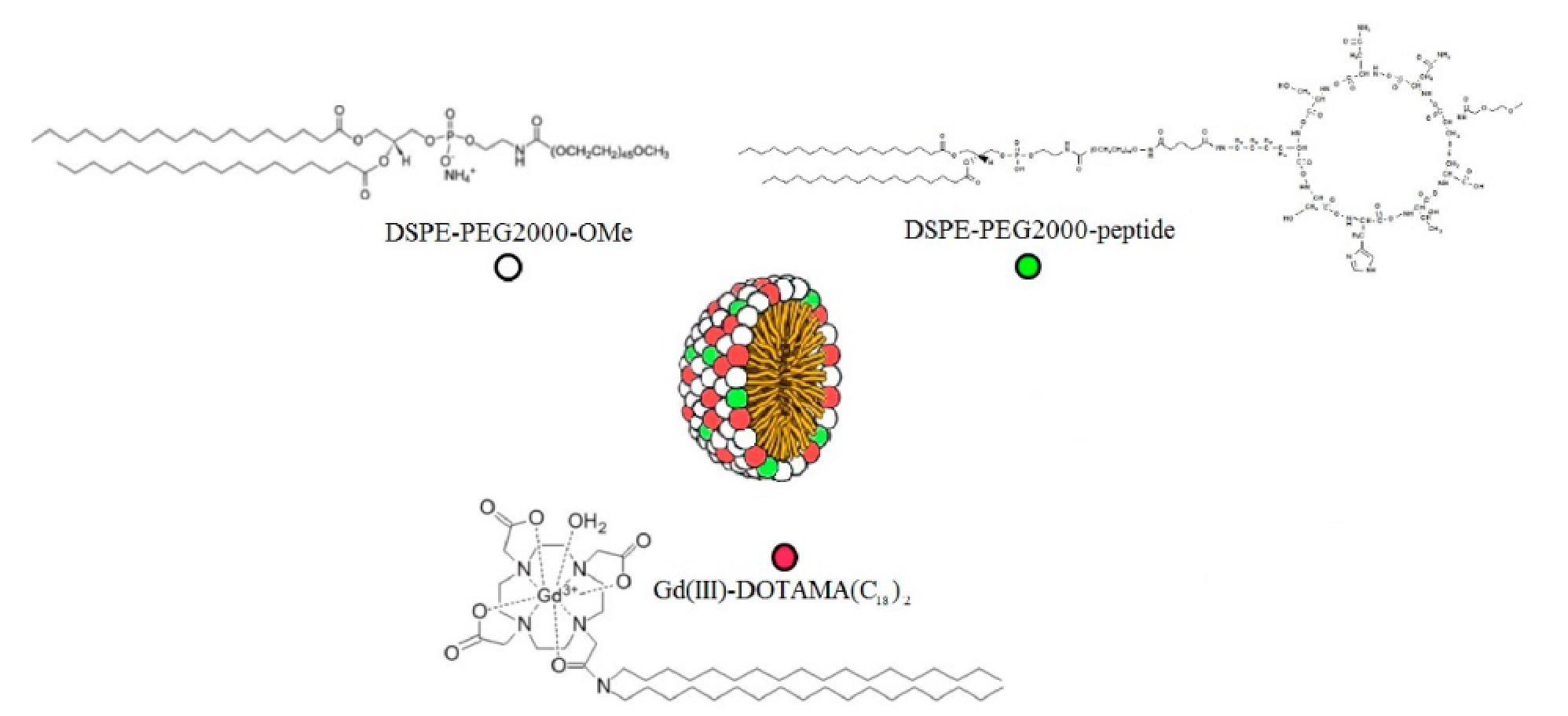
Paramagnetic mixed micelles targeting VCAM-1 displayed a hydrodynamic diameter of 20 nm with a PDI of 0.2. The millimolar longitudinal relaxivity (r1) at 0.5 T was 34.9 s-1mmolGd-1 at 25°C and 35.3 s-1mmolGd-1 at 37°C. The relaxivity was also measured as a function of the proton Larmor frequencies (from 0.01 to 70 MHz):
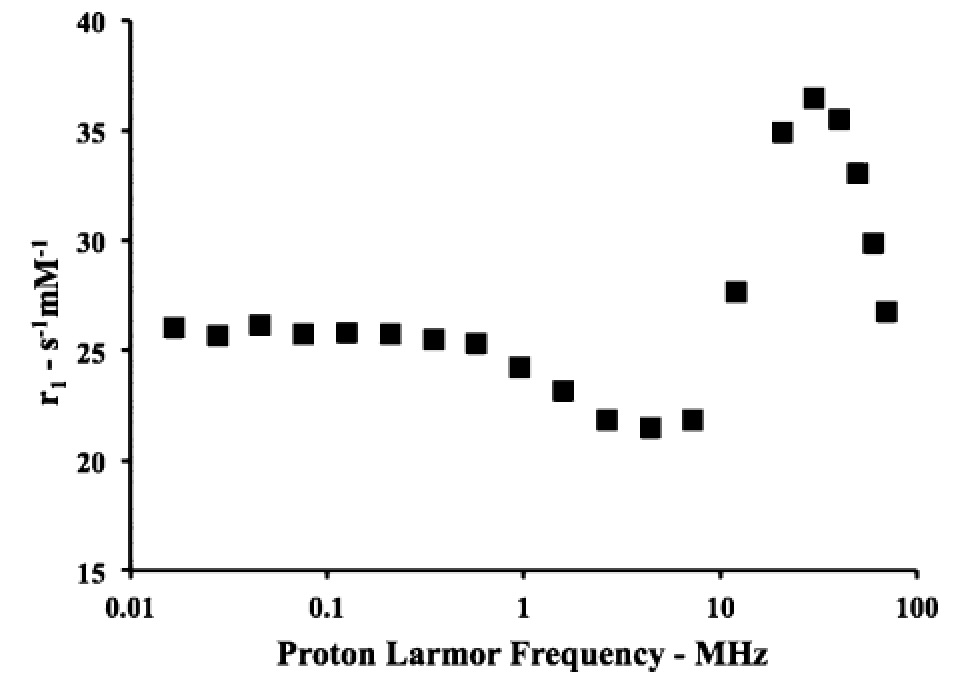
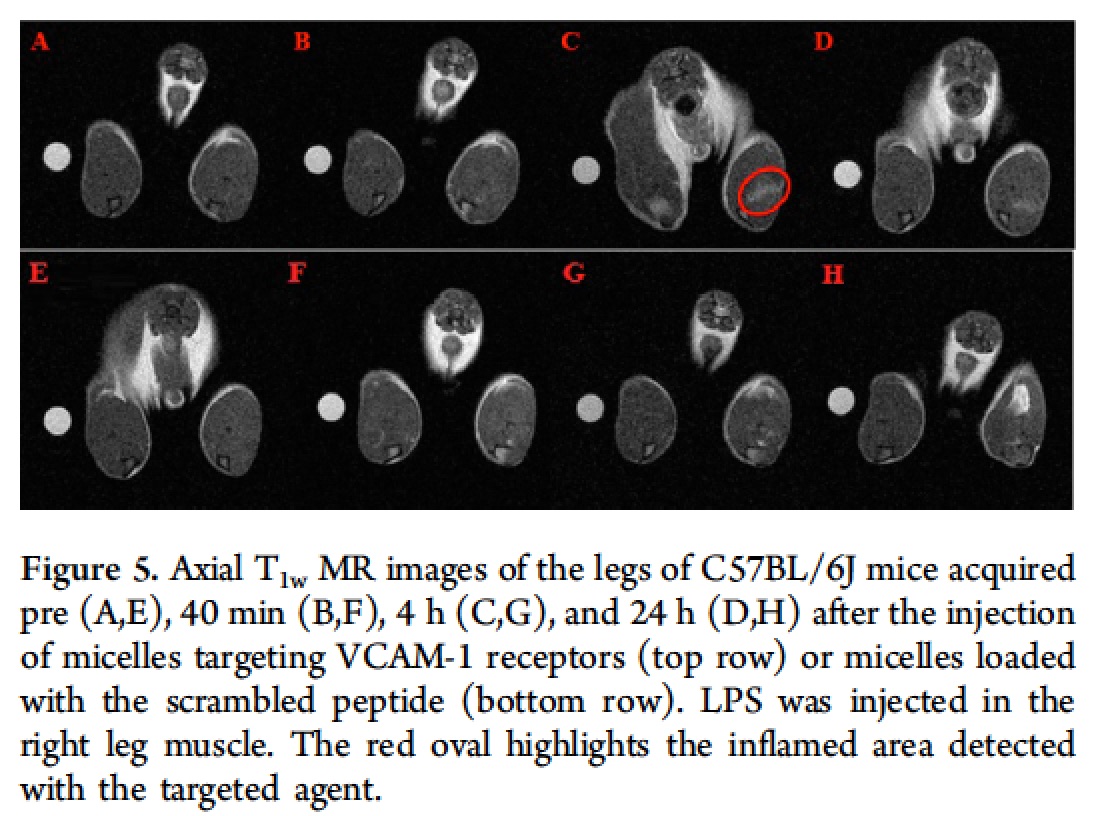
The obtained NMRD (Nuclear Magnetic Relaxation Dispersion) profile is characterized by a relaxivity hump at ca. 20-30 MHz, a feature that clearly indicates the occurrence of a restricted rotational motion for the paramagnetic Gd-complexes in the micelles. The performance of this targeting system has been successfully tested on a mouse model of peripheral inflammation [Pagoto A. et al, 2016], and on a mouse model of neuroinflammation [Garello F. et al, 2017] . The peripheral inflammation was generated by injecting lipopolysaccharide (LPS) in the mouse leg skeletal muscles. Conventional H&E (hematoxylin and eosin) histology confirmed the extensive infiltration of monocytes, lymphocytes, and neutrophils in the leg 48 h after LPS injection. Furthermore, immunofluorescence experiments confirmed the expression of VCAM-1 receptors in the inflamed muscle. Forty-eight hours after the induction of inflammation, the paramagnetic micelles (containing the targeting or a scrambled untargeted peptide) were injected intravenously in the tail vein at a Gd dose of 55.0 µmol/kg bw. Then, the animals (n = 6) were scanned by MRI (at 1 T) over a period of 24 h postinjection.
At T1 contrast enhancement around 45% was observed in the inflamed region 4 h post-injection The contrast measured in the inflamed leg was ca. 5-fold higher than the enhancement detected in the contralateral healthy leg, and 2-fold higher than the response measured upon administration of the control micelles functionalized with the scrambled peptide. The MR images reported below show that 4 h after the injection of the micelles, a hyperintense signal was visible in the inflamed leg (red circle), while no brightening was detected in the control experiment.
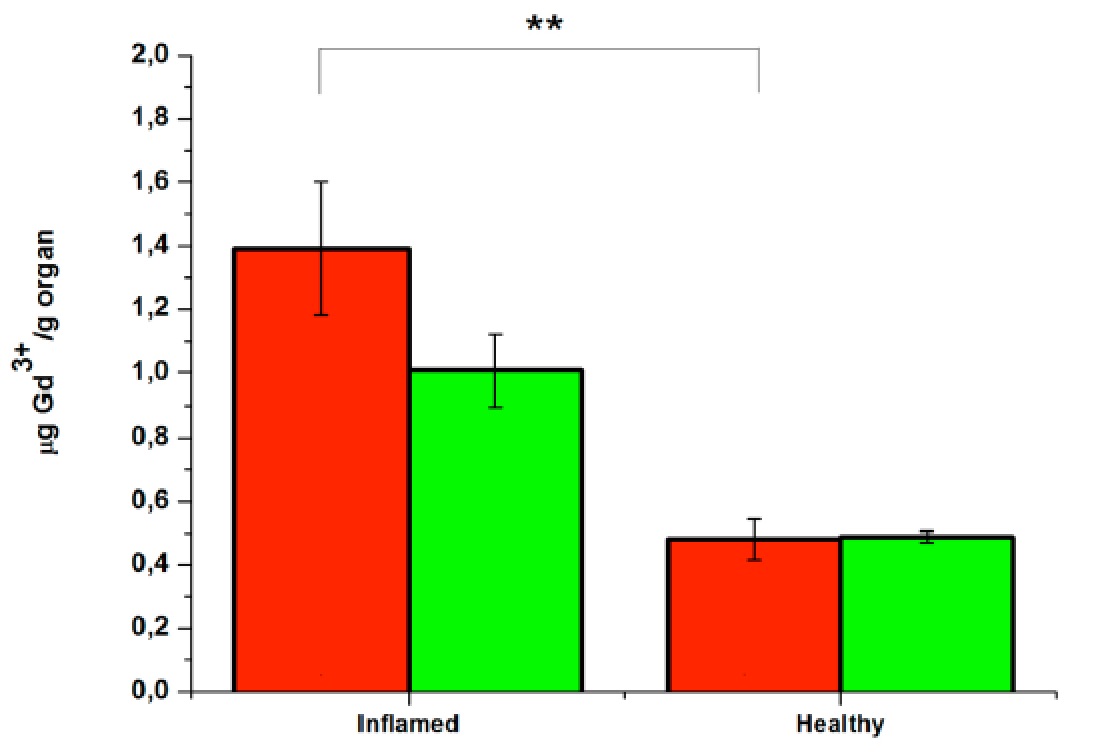
The accumulation of the MRI agent in the legs was confirmed by ICP-MS analysis, which showed that the amount of metal ion in the inflamed muscles after the injection of the VCAM-1-targeted micelles was ca. 40% higher than the amount found in the diseased tissue after the injection of the control micelles loaded with the scrambled peptide and ca. 3-fold higher with respect to the Gd found in the healthy muscles regardless of the type of micelles injected.
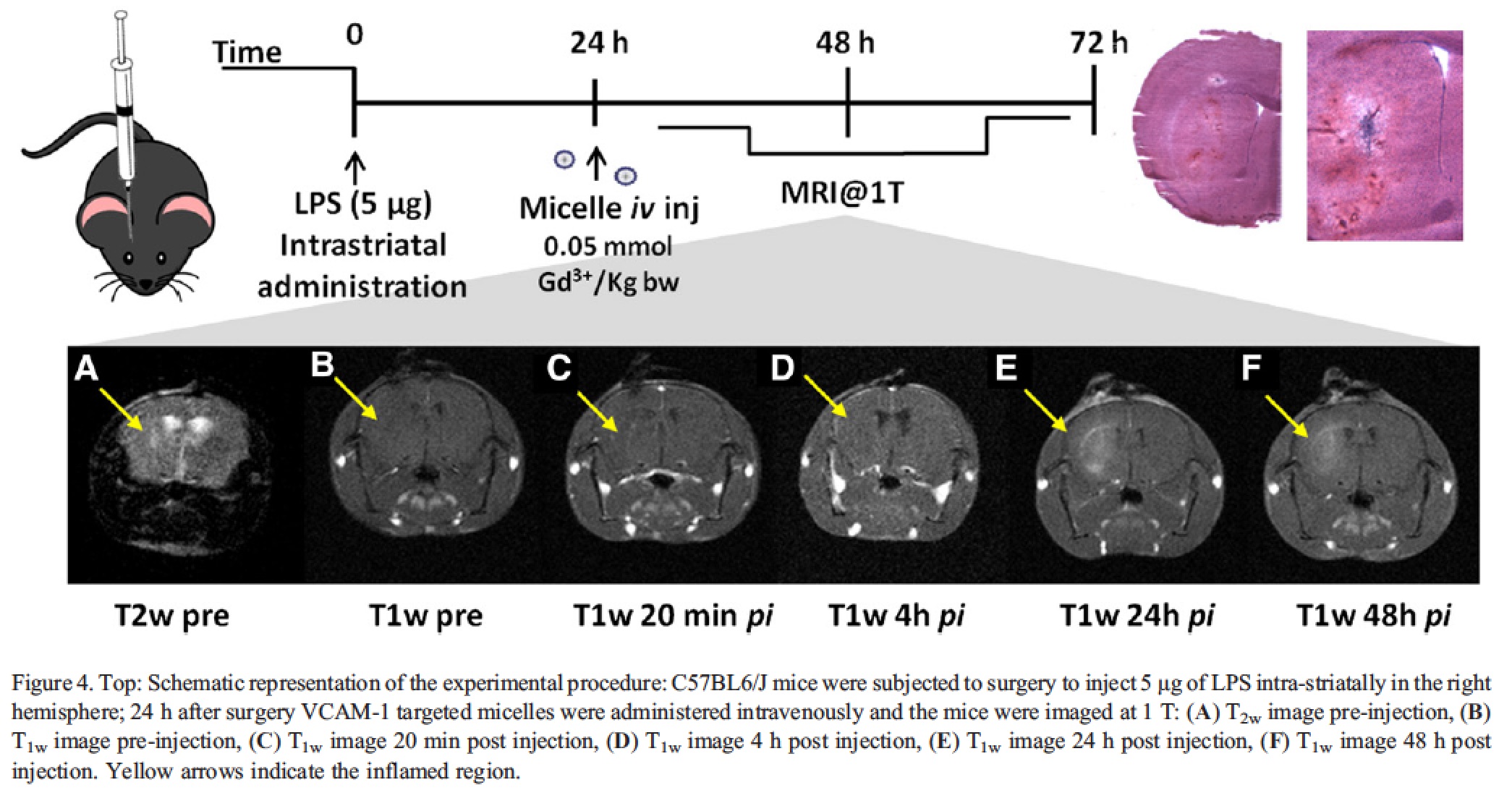
The neuroinflammation model was prepared through a local injection of (LPS) in the right striatum. The presence of inflammation and the over-expression of VCAM-1 in the involved hemisphere were detectable already 24 h after surgery by ex-vivo histology, whereas no hallmarks of inflammation were present in the contra-lateral hemisphere. The animals were enrolled in the study 24 h after LPS injection, received intravenous administration of VCAM-1 targeted micelles (0.05 mmolGd/kg bw) and were imaged at 1.0 T at 20 min, 4 h, 24 h and 48 h post injection to monitor micelle homing to the site of inflammation.
The T1 signal enhancement calculated over pre-images, reached a peak value 24 h post micelle injection, with a statistically significant difference between diseased and healthy hemispheres. To ascertain the specificity of the administered nanosystem, a comparison with micelles bearing the untargeted scrambled peptide was conducted. The results obtained displayed a statistically significant difference in the % T1-SE of the inflamed striatum between targeted and non-targeted micelles at 24 h p.i. (39.3 ± 4.4 vs. 18.9 ± 2.2%, respectively, ANOVA P values = 0.003.
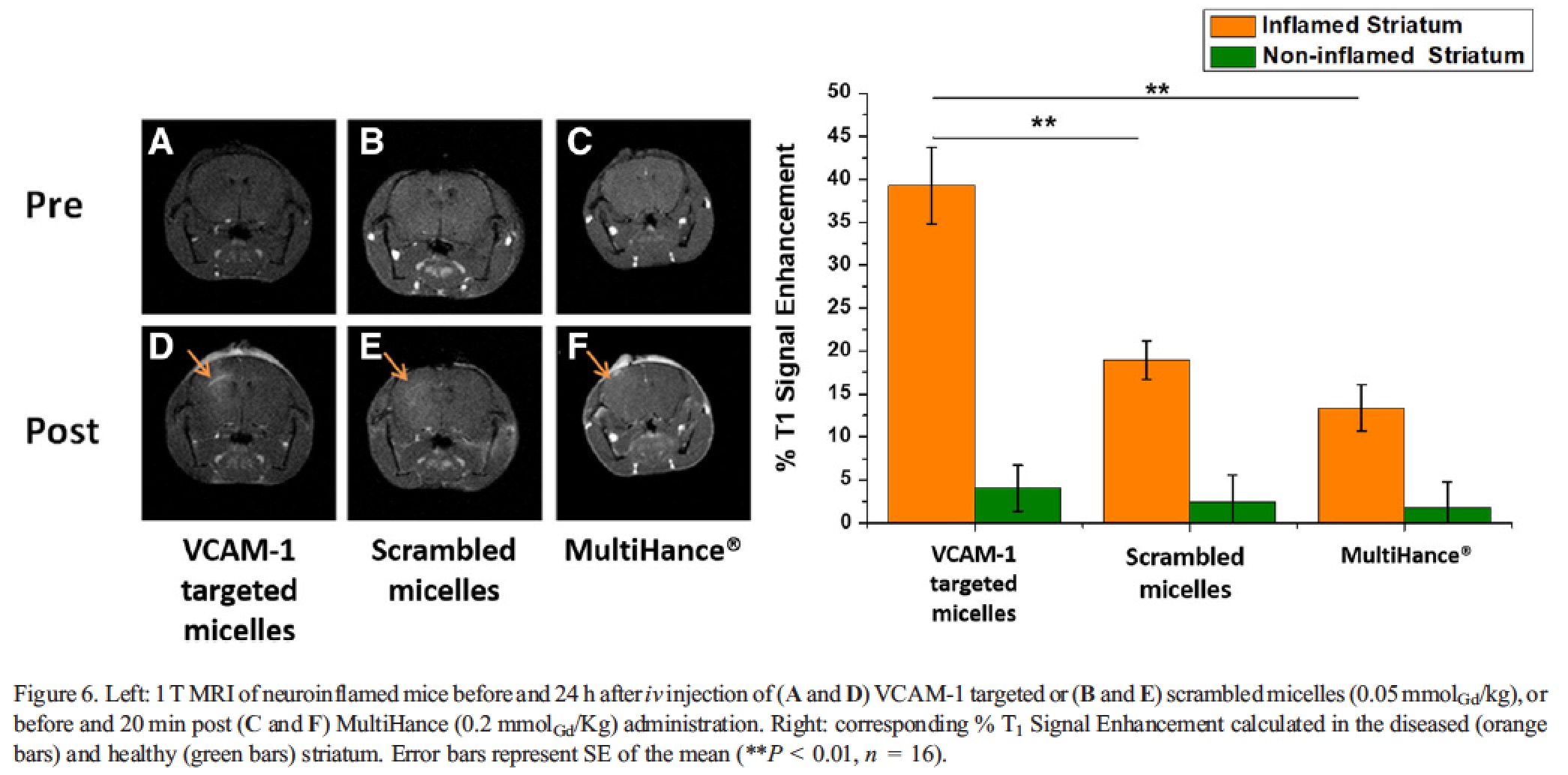
At the other time points, no statistically significant differences between the two nanosystems were detected. Interestingly, the kinetic of the contrast enhancement observed for the two nanosystems was a bit different, with the targeted system that performed much better after 24 h post-injection, whereas at 48 h the two micelles showed a very similar effect. Most likely, this observation is an indirect demonstration of the effective targeting of the VCAM-1 directed system, and it suggests that the targeting to the receptor (though, likely, it is not the only process accounting for the accumulation of the probe in the inflamed area) is a faster event than the passive accumulation occurring for the untargeted micelles. The signal obtained 24 h post injection of the scrambled micelles was definitely comparable to the T1-SE detected 20 min after the administration of the contrast agent MultiHance (13.4% ± 2.7%), which is clinically employed to evaluate the presence of alterations in blood brain barrier permeability. This finding suggests that the contrast obtained after the administration of scrambled micelles is mainly related to passive extravasation, thus further supporting the view that VCAM-1 targeted micelles effectively bind the corresponding target in vivo.