Development of cellular imaging procedures
Tracking cells in vivo by using imaging approaches represents a reliable method to assess the characteristics of cell grafts and to monitor their fate after transplantation. In that respect, minimally invasive techniques with high spatial resolution are desirable with a view of implementing therapeutic protocols in the clinical ordinary. MRI is a leading imaging modality enabling the non-invasive visualization of cell populations and their movements after transplantation in living animals with superb resolution. In order to be detectable, cells require to be adequately labelled with MRI contrast agents (CAs). Due to their excellent imaging efficiency, the superparamagnetic iron oxide nanoparticles (SPIONs) are regarded as the gold standard in cell-labeling. However, some issues are associated with their contrast generation mechanism based on signal-loss (negative contrast). As a promising alternative, paramagnetic CAs based on the metal Gadolinium (Gd) create a contrast increment (positive contrast) in T1-weighted (T1w) images, overcoming several complications related to the use of SPIONs. Even if toxic in the form of free aqueous ion, Gadolinium is generally considered safe when administered as a macrocyclic chelate and several agents have been approved for the clinical use so far. Differently from magnetic nanoparticles, large amounts of paramagnetic small molecules have to be delivered to target sites in order to be detected, and several labeling procedures have been developed to achieve this goal. In addition to Gd(III)-complexes, also fluorinated nanoparticles represent a valuable option for cellular imaging, especially for the visualization of the macrophage recruitment in inflammation sites or to tracking dendritic cells. One of the advantages of 19F-MRI is the possibility to quantify the signal and calculate the cellular content in the site of interest. Besides MRI, other imaging modalities can be used for tracking cells in vivo, like optical or photoacoustic imaging.
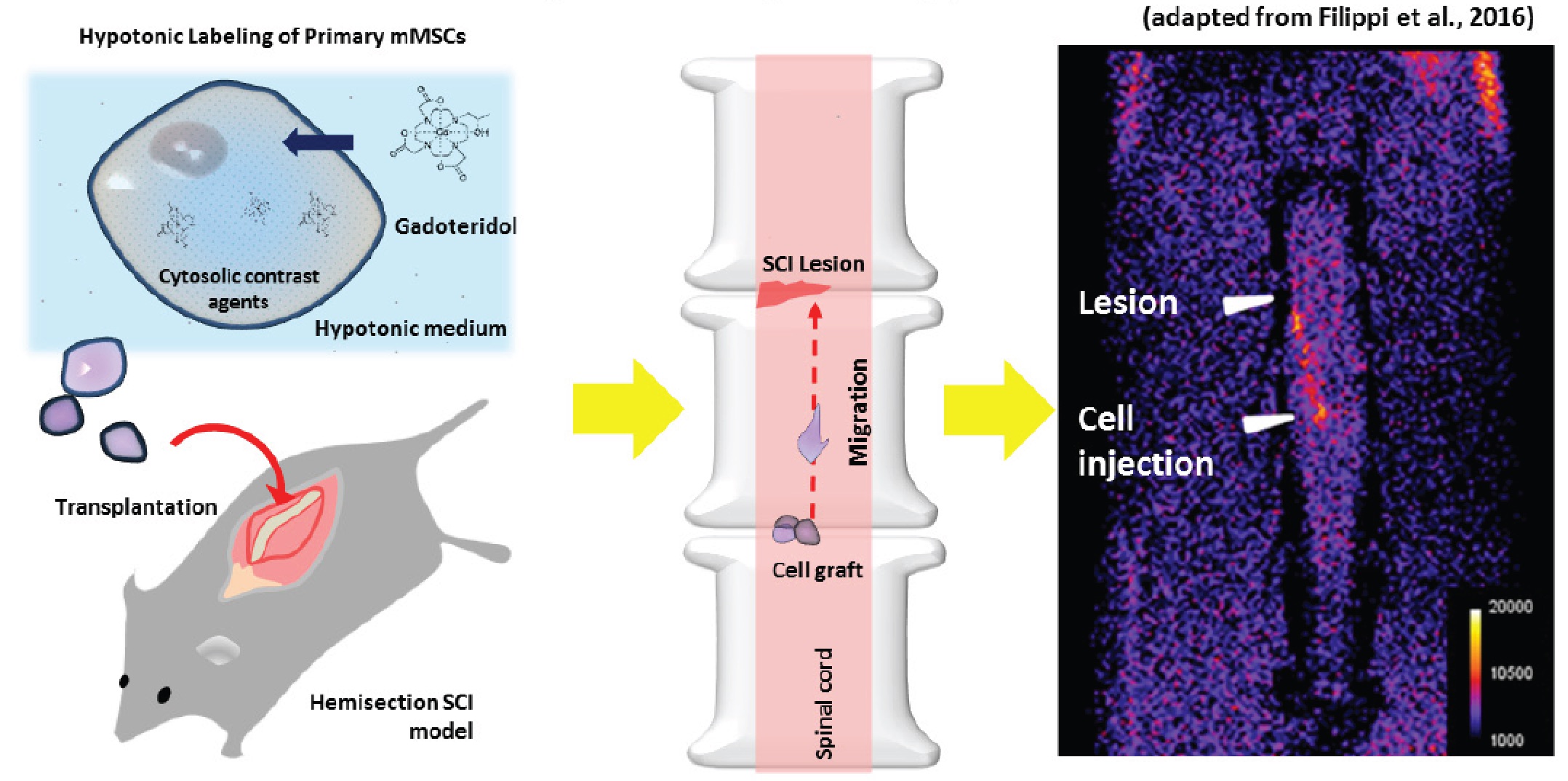
MRI tracking of MSCs labeled with Gadoteridol in a Spinal Cord Injury experimental model
In this activity, Filippi M. et al.,
2016 labelled murine Mesenchymal Stem Cells (MSCs)
with the clinically approved MRI agent Gadoteridol through a
procedure based on the hypo-osmotic shock. Cells were
successfully tracked in vivo in a murine model of Spinal Cord
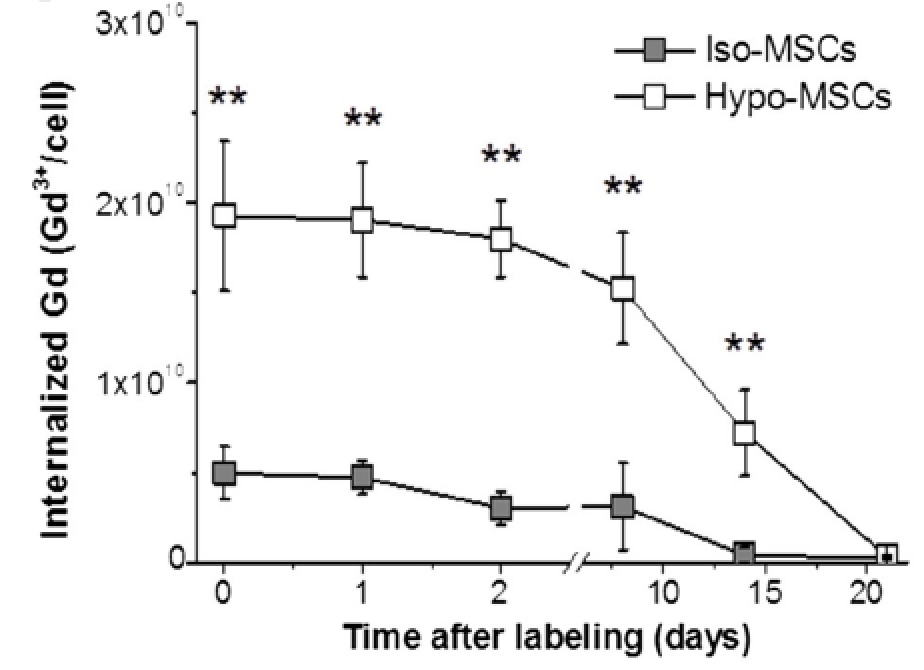
Injury (SCI). 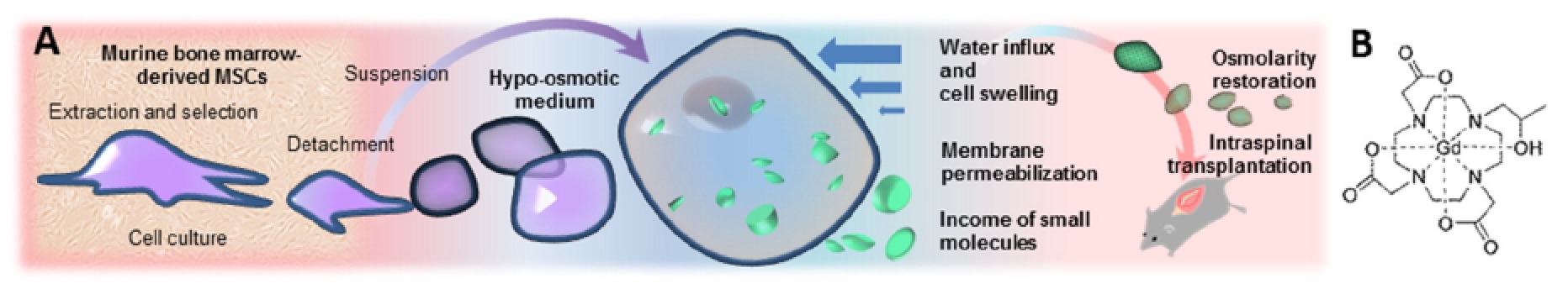 The hypo-osmotic shock method Di Gregorio E. et
al, 2013 allowed the internalization of a higher
number of contrast agent units than the conventional isotonic
labeling.
The hypo-osmotic shock method Di Gregorio E. et
al, 2013 allowed the internalization of a higher
number of contrast agent units than the conventional isotonic
labeling.
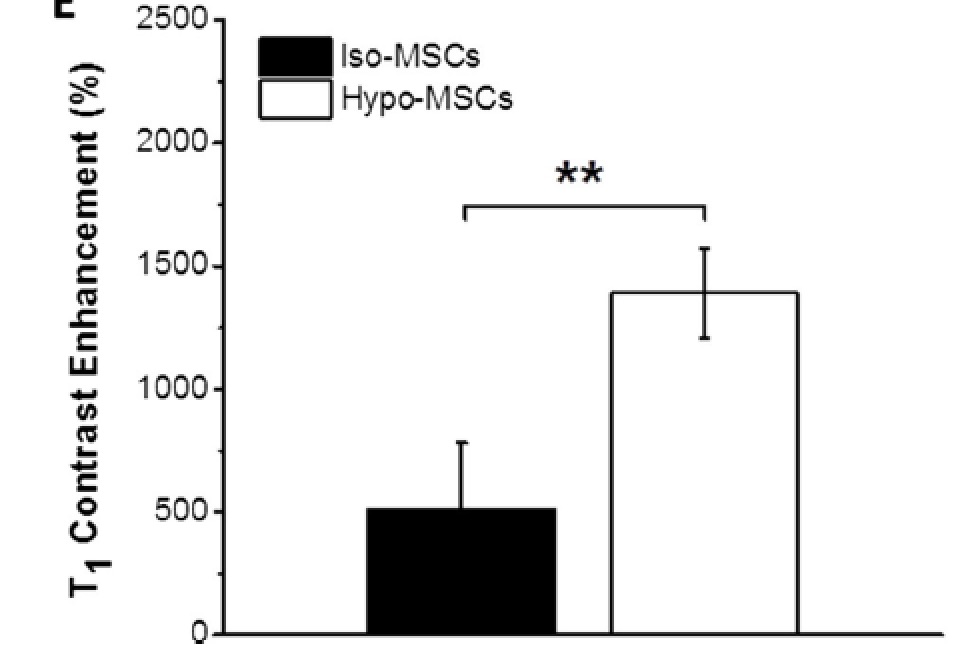
Furthermore, also the T1 contrast enhancement of Gadoteridol internalized using the hypo-tonic method is higher than the isotonic technique.
The differences observed in vitro were confirmed in vivo after transplantation of a different number of cells in a mouse model of spinal cord injury.
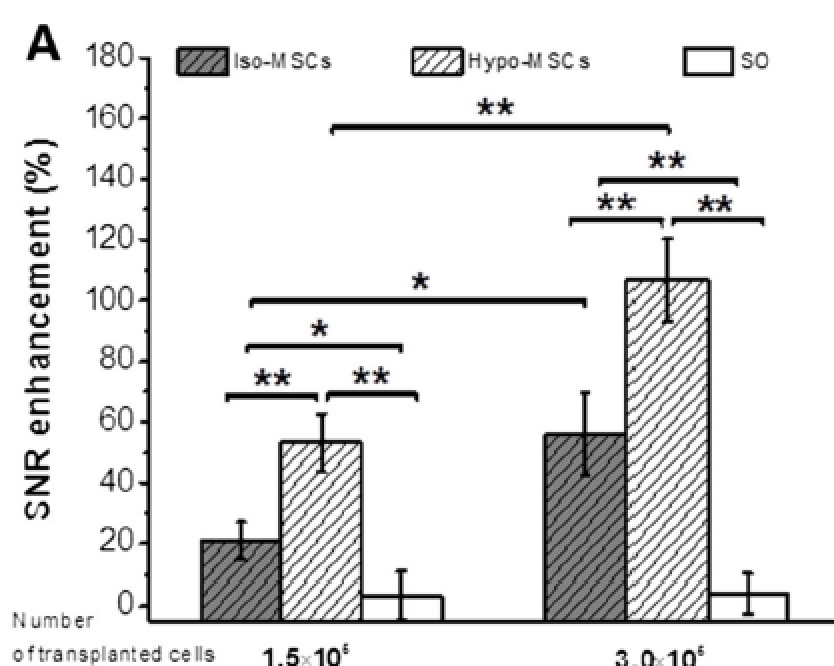
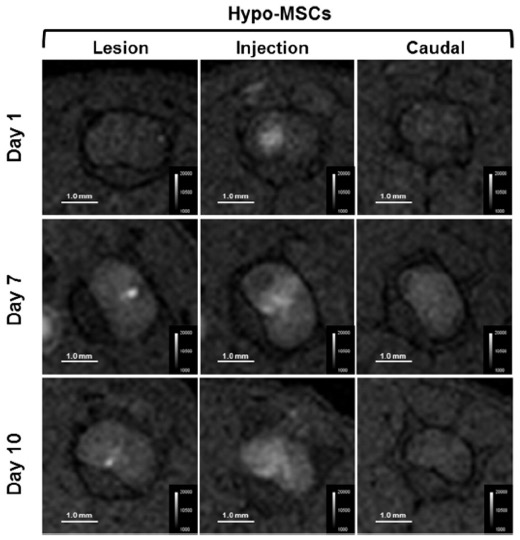
Interestingly, the injected cells migrated from the injection site to the lesion site, and MRI was able to visualize this movement.
 Very important, the diseased mice treated with the labelled
cells displayed an excellent recover from the pathology, as
demonstrated by carrying out behavioural tests.
Very important, the diseased mice treated with the labelled
cells displayed an excellent recover from the pathology, as
demonstrated by carrying out behavioural tests.
Fluorinated nanoparticles for the in-vivo tracking of inflammation in a mouse model of spinal cord injury
The same model has been also used to assess the macrophagic
infiltrate after the onset of the lesion (Garello F. et al,
manuscript in preparation, presented at WMIC 2017). The method
consisted of injecting in the mice a per-fluoro nanoemulsion
based on perfluoro-15-crown-5-ether (PFCE-NE). These
nanoparticles are rapidly taken up by circulating monocytes
that bring them in the inflammation site. PFCE-NE suspension
contained particles with a hydrodynamic size of 170±20nm (PDI
0.07) with a total fluorine concentration of around 3.4 M. The
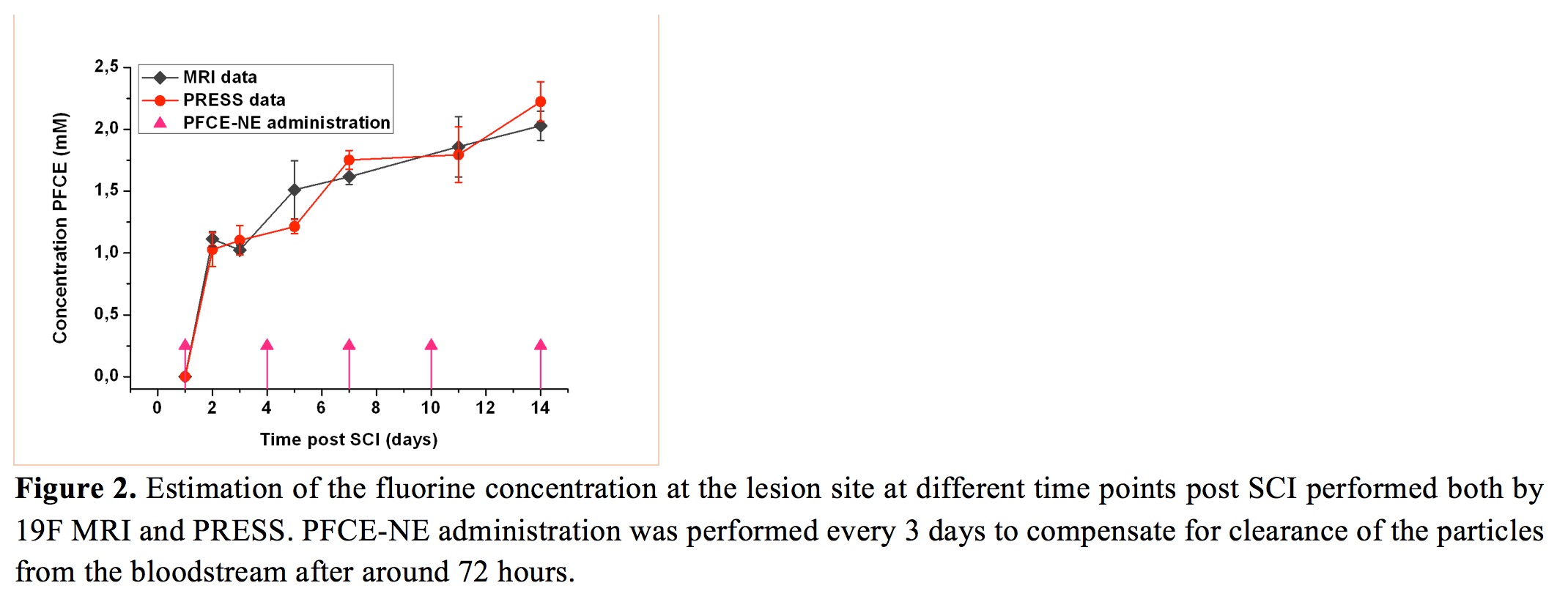
recruitment of macrophages at the lesion site was successfully
followed for 2 weeks, both by 19F-PRESS and MRI, displaying the
higher signal enhancement in the first days post injury (pi)
even if a constant and faint fluorine accumulation was
detectable throughout the 14 days of monitoring. At days 2 and
14 pi, the amount of fluorine at the lesion was 0.9 µmol and
1.7 µmol, respectively. Ex-vivo validation by IF displayed a
massive accumulation of particles at the lesion site, mainly
phagocytized by peripheral and resident immune cells. 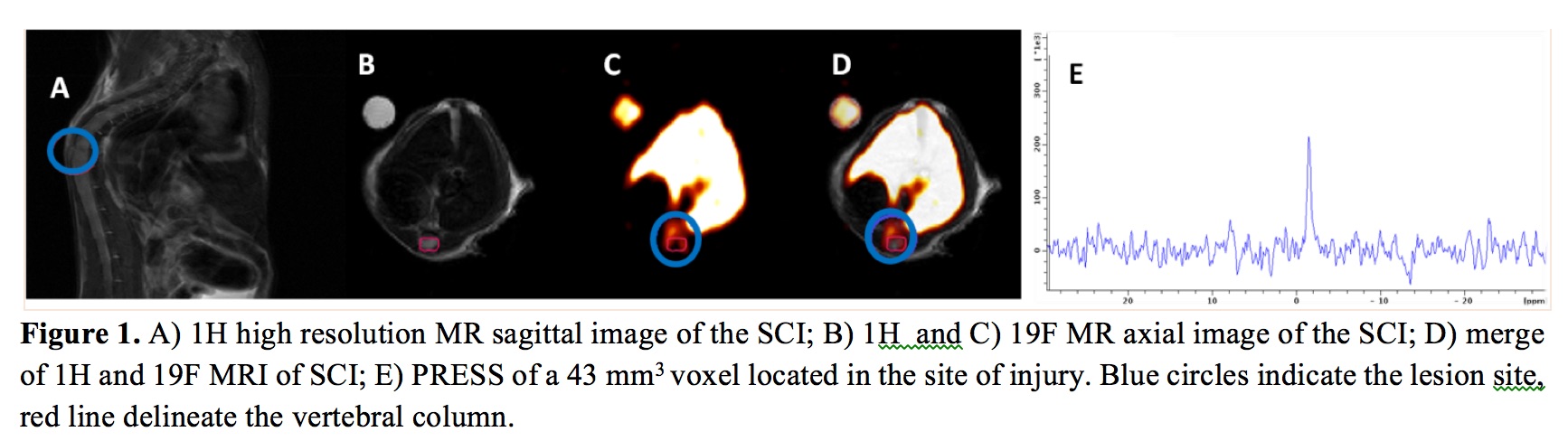
Indocyanine Green labeling for optical and photoacoustic imaging of Mesenchymal Stem Cells after in vivo transplantation
In this activity [Filippi M. et al. 2018], the potential of NIRF and Photoacoustic imaging in cellular imaging has been assessed labeling Mesenchymal Stem Cells with the clinically approved dye indocyanine green (ICG). Labelled cells were subsequently transplanted in healthy mice. The in vivo study was carried out by locally transplanting 3.0×105 ICG-labeled MSCs into the gastrocnemius muscle of the right hindlimb of healthy C57BL/6J mice. The site of cell deposition was clearly detected by US imaging (B-mode, 21 MHz), as a consequence of the change in the acoustic impedance determined by the dense inoculated cell mass. The PA signal intensity generated by the ICG-MSCs was normalized over the local endogenous baseline recorded in the left hindlimb of the animal where the transplantation of control unlabelled cells was performed.
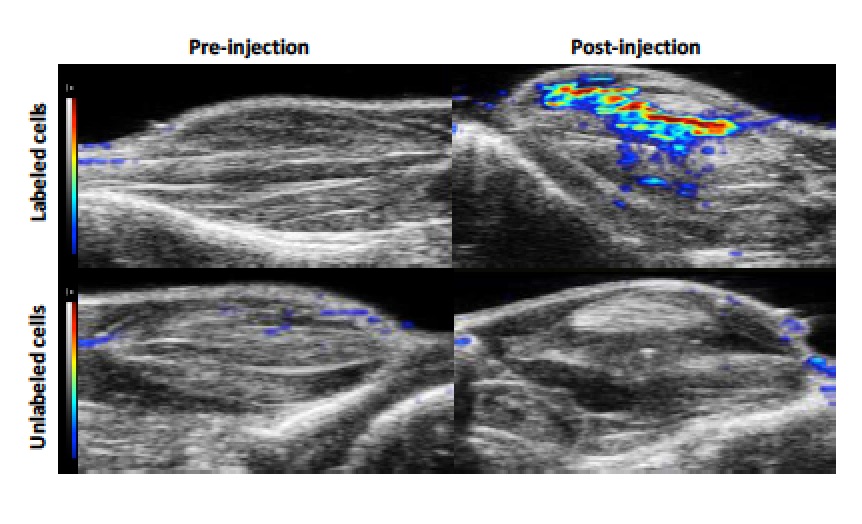
The PAEnh was measured over the entire range of excitation wavelengths immediately after cell transplantation, then monitored over time, and reported as PA spectrum.
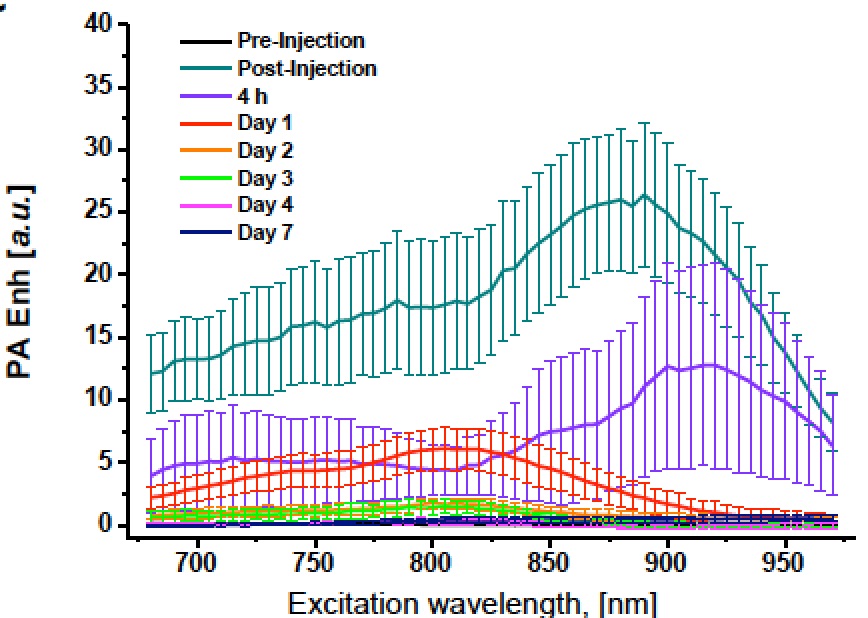
Interestingly, immediately and 4 hours post-injection, the maximum peak recorded in the photoacoustic spectra was shifted towards high excitation wavelength values (890 and 920 nm, respectively), whereas from day 1 to day 4, the spectral shape reproduced the one observed in vitro with a maximum enhancement centred at around 810 nm. The normalized PA spectra acquired 7 days post injection presented a flat shape without any discernable peak. After each PA acquisition, the mice underwent NIRF imaging in order to assess the fluorescent contrast enhancement (FLIEnh) produced by the transplanted cells. Interestingly, before progressively fading over days (likely due to the dye degradation and washout), the FLIEnh values followed an initial rising trend during the first 24 h after the engraftment deposition.
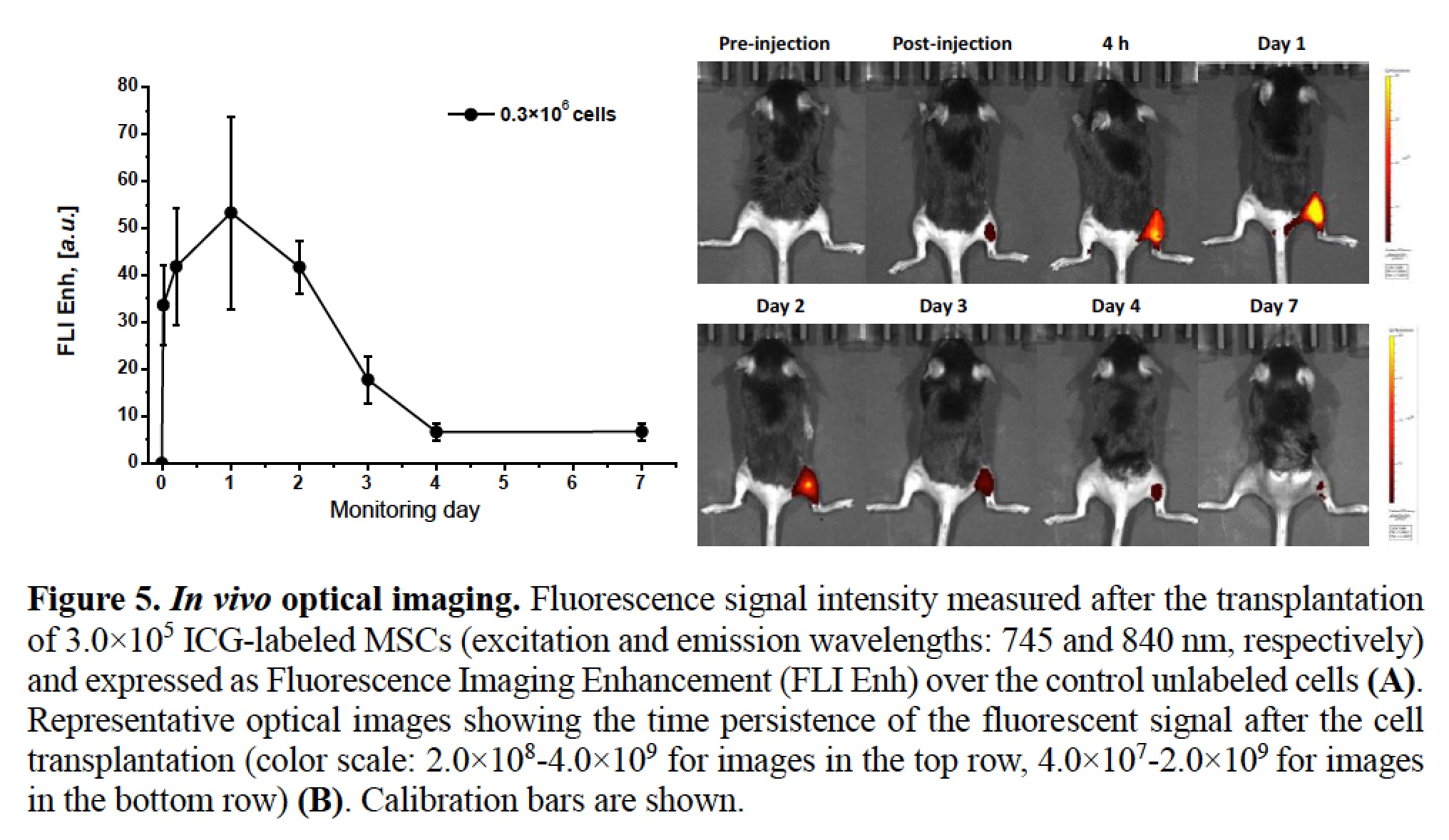
The coincident observation of extremely high PA amplitudes suggests that at early time points the strong intermolecular interactions among ICG molecules contribute to the quenching of the fluorescence, but results at the same time into an increase of the photoacoustic effect, due to photothermal conversion by nonradiative decay.