Investigating tumor pH imaging as a novel cancer biomarker
Tumor cells that live in conditions of hypoxia show an up-regulated glucose metabolism, because of hypoxia-induced shift toward
glycolysis. Tumor cells have evolved several sophisticated mechanisms to regulate pH homeostasis: they eliminate acidic catabolites
by ion transporters and pumps to preserve a slightly alkaline intracellular pH (pHi), which is optimal for cell proliferation and
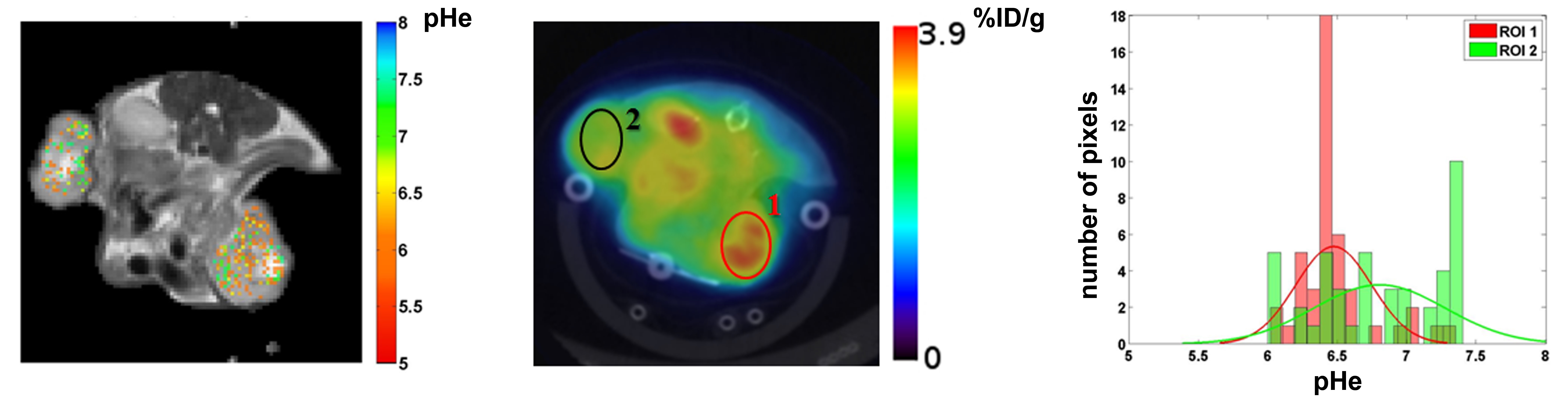
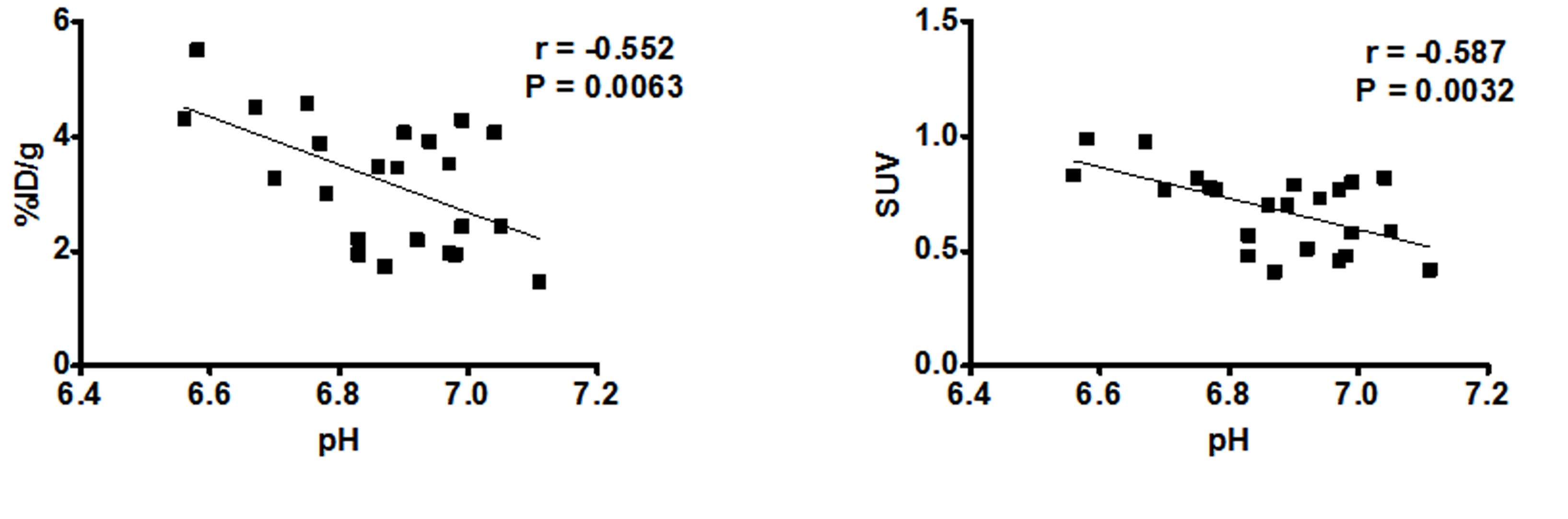
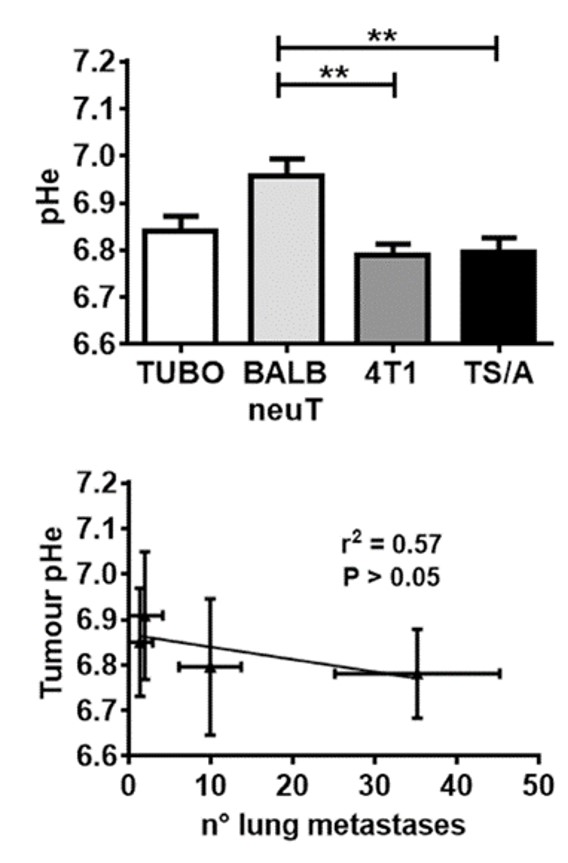
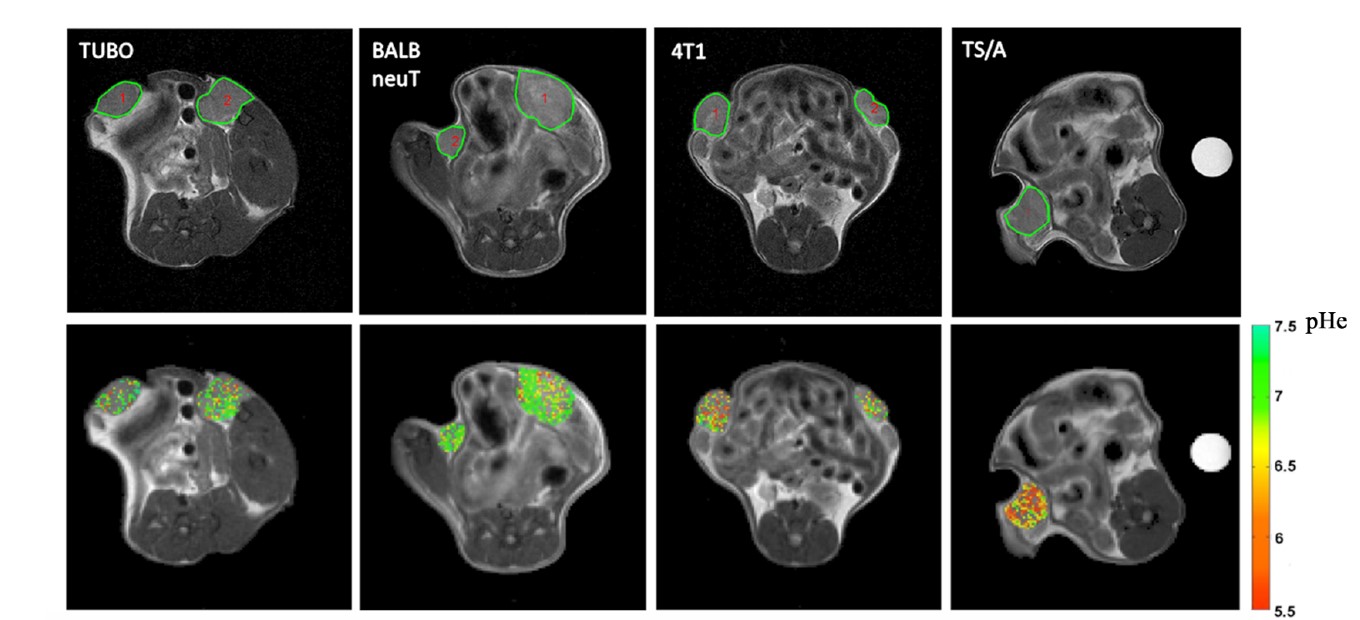
tumor survival.This behavior leads to enhanced acidification of the extracellular pH (pHe) to values in the range 6.5-7.0, which is
a distinguishing feature of the tumor microenvironment. Imaging based methods have already been established, at a clinical level,
to assess glucose metabolism (by positron emission tomography - PET imaging of 18F-fluorodeoyglucose FDG tumor uptake), providing a
formidable tool for evaluating treatment response.
Conversely, despite the excellent studies regarding tumor acidosis, we still
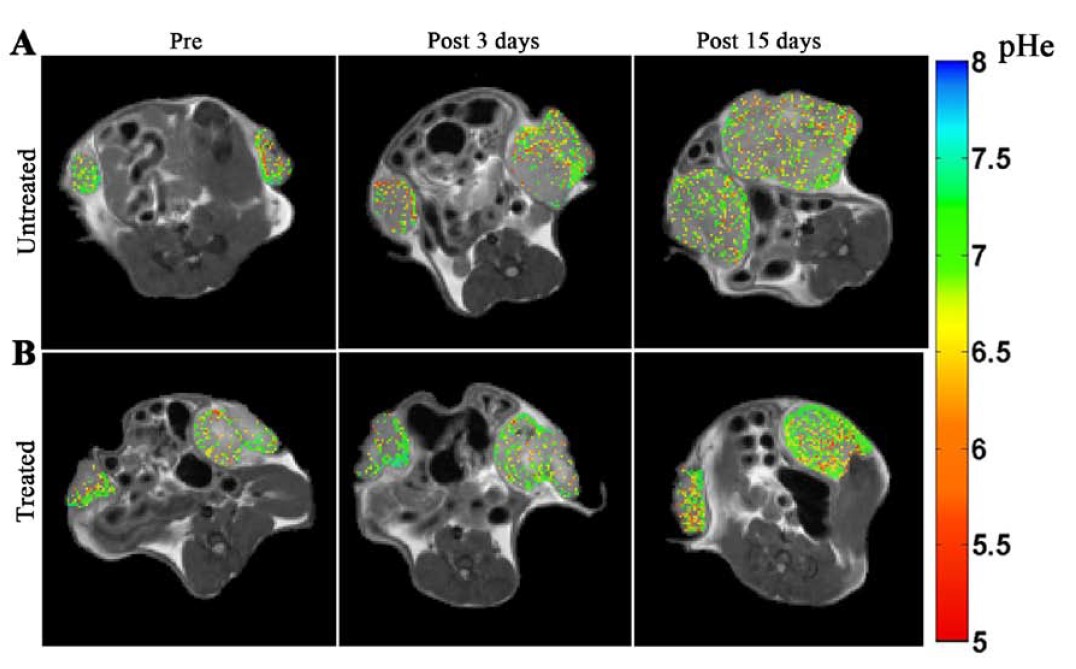
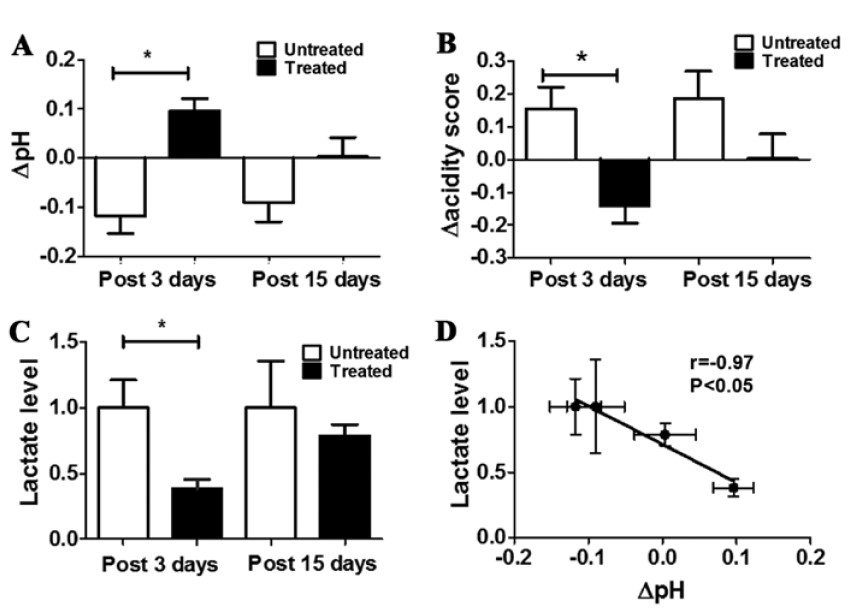
do not have an effective acid-base imaging protocol that allows the quantification of extracellular tumor pH and the assessment
of pHe related changes following therapeutic treatment.
The focus of our research is to develop non invasive MRI-based tools to assess
tumor pHe and to investigate tumor pHe heterogeneity as a potential innovative biomarker for evaluating treatment response to drugs targeting tumor metabolism.